How is KOVALTRY® packaged and stored?
KOVALTRY® is available with the Vial Adapter needleless reconstitution system, which contains1:
Vial Adapter with built-in 15-micrometer filter
2.5 mL or 5.0 mL prefilled diluent syringe
25-gauge butterfly needle

KOVALTRY® comes in a wide range of vial sizes1
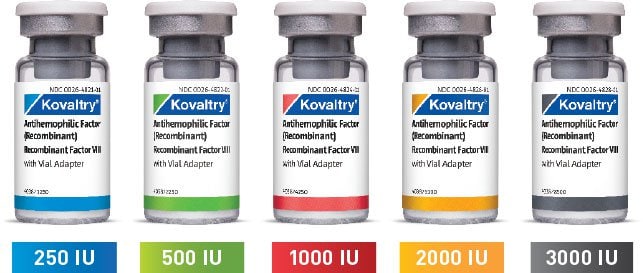
250 IU, 500 IU, and 1000 IU with 2.5 mL prefilled diluent syringe
2000 IU and 3000 IU with 5.0 mL prefilled diluent syringe
How to store KOVALTRY®1
At room temperature:
KOVALTRY® can be stored at room temperature (up to 77°F) for up to 1 year
When storing KOVALTRY® at room temperature, the starting date of storage should be clearly recorded on the unopened product carton
Once stored at room temperature, KOVALTRY® must not be returned to the refrigerator
Stored at room temperature, the shelf life of KOVALTRY® expires after 12 months or at the expiration date on the KOVALTRY® vial, whichever is earlier
In the refrigerator:
Do not freeze
KOVALTRY® can be stored in the refrigerator (36°F to 46°F) for up to 30 months from the date of manufacture
Once KOVALTRY® is removed from the refrigerator, it must not be returned to the refrigerator
Please see full Prescribing Information for complete storage and handling instructions.

At room temperature:
KOVALTRY® can be stored at room
temperature (up to 77°F) for up to 1 year

When storing KOVALTRY® at room temperature, the starting date of storage should be clearly recorded on the unopened product carton
Once stored at room temperature, KOVALTRY® must not be returned to the refrigerator
Stored at room temperature, the shelf life of KOVALTRY® expires after 12 months or at the expiration date on the KOVALTRY® vial, whichever is earlier
In the refrigerator:
Do not freeze
KOVALTRY® can be stored in the refrigerator (36°F to 46°F) for up to 30 months from the date of manufacture
Once KOVALTRY® is removed from the refrigerator, it must not be returned to the refrigerator
Please see full Prescribing Information for complete storage and handling instructions.
INDICATION FOR KOVALTRY®
KOVALTRY® is a medicine used to replace clotting factor (Factor VIII or antihemophilic factor) that is missing in people with hemophilia A.
KOVALTRY is used to treat and control bleeding in adults and children with hemophilia A. KOVALTRY can reduce the number of bleeding episodes in adults and children with hemophilia A when used regularly (prophylaxis). Your healthcare provider may give you KOVALTRY when you have surgery.
KOVALTRY is not used to treat von Willebrand Disease.
IMPORTANT SAFETY INFORMATION
You should not use KOVALTRY if you are allergic to rodents (like mice and hamsters) or any ingredients in KOVALTRY.
Tell your healthcare provider if you have heart disease or are at risk for heart disease.
The common side effects of KOVALTRY are fever, headache, and rash, in addition to inhibitors in patients who were not previously treated or minimally treated with Factor VIII products.
Your body may make antibodies, called “inhibitors” against KOVALTRY, which may stop KOVALTRY from working properly. If your bleeding is not adequately controlled, it could be due to the development of Factor VIII inhibitors. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to Factor VIII.
Allergic reactions may occur with KOVALTRY. Call your healthcare provider right away and stop treatment if you get tightness of the chest or throat, dizziness, decrease in blood pressure, and nausea.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your healthcare provider right away if bleeding is not controlled after using KOVALTRY.
For additional important risk and use information, please see full Prescribing Information.
Reference: 1. KOVALTRY® [prescribing information]. Whippany, NJ: Bayer HealthCare LLC; 2021.


