How is KOVALTRY® prepared and taken?
KOVALTRY® is available with the Vial Adapter reconstitution system1
Learn how to use KOVALTRY® with Vial Adapter
Download printable instructions for using KOVALTRY®
Learn how to use KOVALTRY® with Vial Adapter
Download printable instructions for using KOVALTRY®
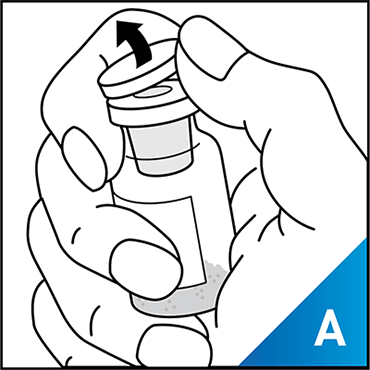
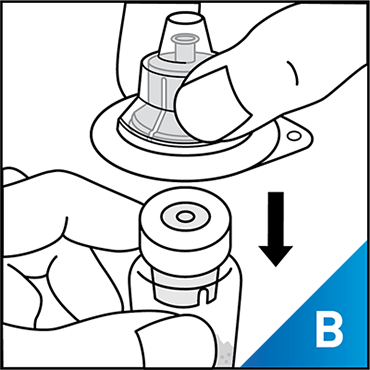
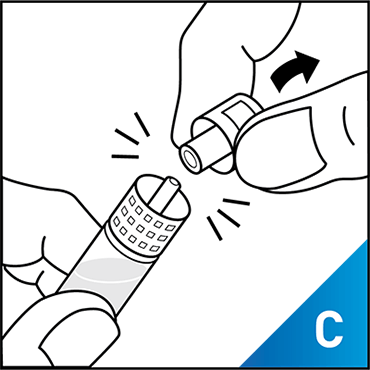
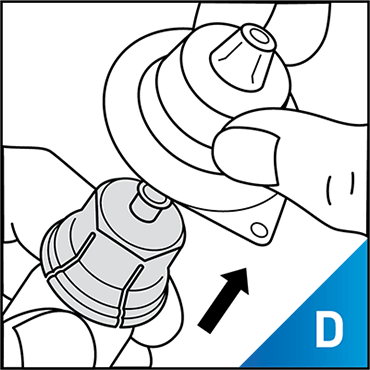
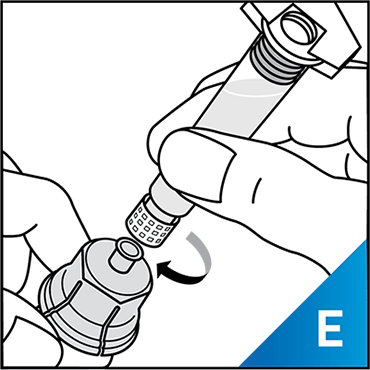
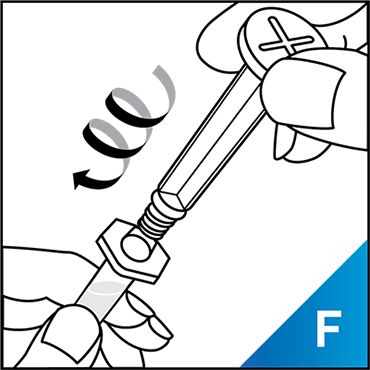
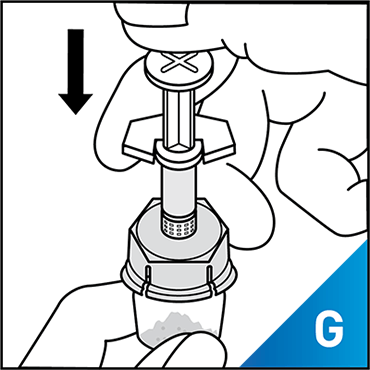
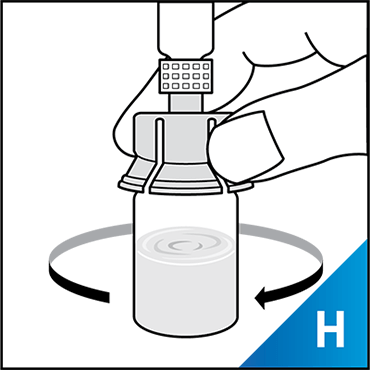
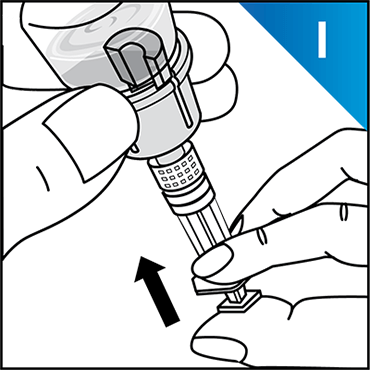
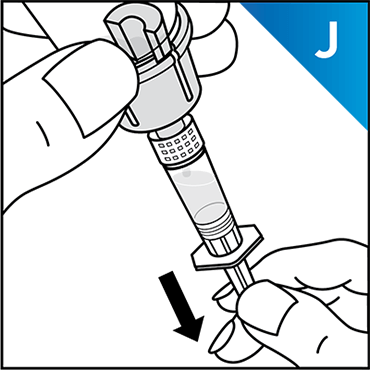
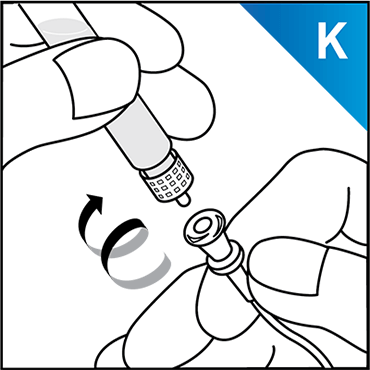
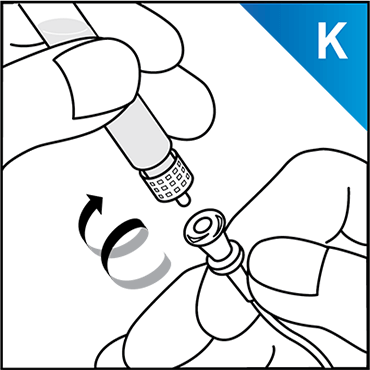
Always work on a clean surface and wash your hands before performing the following procedure. Use only the components for reconstitution and administration that are provided with each package of KOVALTRY®. If a package is opened or damaged, do not use this component. If these components cannot be used, please contact your healthcare provider.
Prepare a clean flat surface and gather all the materials needed for the infusion.
Always work on a clean surface and wash your hands before performing the following procedure. Use only the components for reconstitution and administration that are provided with each package of KOVALTRY®. If a package is opened or damaged, do not use this component. If these components cannot be used, please contact your healthcare provider.
Prepare a clean flat surface and gather all the materials needed for the infusion.
Click here to learn how to avoid potential errors when using Vial Adapter
Pooling
If the dose requires more than one vial, reconstitute each vial as described above with the diluent syringe provided. To combine the content of the vials, use a larger plastic syringe (not provided) to pool the solution into the syringe and administer as usual.
Rate of Administration
The entire dose of KOVALTRY® can usually be infused within 1 to 15 minutes. Your healthcare provider will determine the rate of administration that is best for you.
INDICATION FOR KOVALTRY®
KOVALTRY® is a medicine used to replace clotting factor (Factor VIII or antihemophilic factor) that is missing in people with hemophilia A.
KOVALTRY is used to treat and control bleeding in adults and children with hemophilia A. KOVALTRY can reduce the number of bleeding episodes in adults and children with hemophilia A when used regularly (prophylaxis). Your healthcare provider may give you KOVALTRY when you have surgery.
KOVALTRY is not used to treat von Willebrand Disease.
IMPORTANT SAFETY INFORMATION
You should not use KOVALTRY if you are allergic to rodents (like mice and hamsters) or any ingredients in KOVALTRY.
Tell your healthcare provider if you have heart disease or are at risk for heart disease.
The common side effects of KOVALTRY are fever, headache, and rash, in addition to inhibitors in patients who were not previously treated or minimally treated with Factor VIII products.
Your body may make antibodies, called “inhibitors” against KOVALTRY, which may stop KOVALTRY from working properly. If your bleeding is not adequately controlled, it could be due to the development of Factor VIII inhibitors. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to Factor VIII.
Allergic reactions may occur with KOVALTRY. Call your healthcare provider right away and stop treatment if you get tightness of the chest or throat, dizziness, decrease in blood pressure, and nausea.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your healthcare provider right away if bleeding is not controlled after using KOVALTRY.
For additional important risk and use information, please see full Prescribing Information.
Reference: 1. KOVALTRY® [prescribing information]. Whippany, NJ: Bayer HealthCare LLC; 2021.